If you are interested in home oral ketamine therapy or nasal spray ketamine, contact NOVA Health Recovery Ketamine Center at 571-999-2118 or 703-844-0184 to start treatment. You can also fill out the web form below. Please include your phone number so we can contact you.
Oral ketamine can be an effective option in the treatment of depression and mood disorders.
- It has a significant antidepressant and anti-suicidal effect.
- Studies have not shown much abuse potential or adverse effects.
- It is cheap, convenient, and available.
- It is slower in onset than Intravenous formulations. It produces lower remission and response rates overall.
- It can be used to augment traditional antidepressant therapy.
How is ketamine administered?
- Oral
- Sublingual
- Transmucosal
- Intravenous
- Intramuscular
- Subcutaneous
- Intranasal
- Rectally
Intravenous ketamine is the most studied and demonstrates the best efficacy in mood disorders because it is 100% bioavailable. (4)(5) The dose of IV ketamine is 0.5 mg/kg over 40 minutes. The equivalent dose of oral ketamine would be 120-150 mg (2 mg/kg) in a standard adult.
Oral ketamine is only 20% bioavailable to the blood stream. (1)(2)
Oral ketamine is metabolized in the liver, which results in norketamine elevation (an active breakdown product). (2)
A possible way to increase availability of oral ketamine is to increase the dose.
Support for the use of oral ketamine comes from:
- Case reports
- Chart reviews
- Randomized controlled trials
- Case series
Oral ketamine can augment traditional antidepressant therapies:
In the study above, an RCT was performed in which 81 patients were followed with the combination of oral ketamine and sertraline or sertraline alone and monitored with the Hamilton Depression Rating Scale (HDRS) at baseline, 2,4 and 6 weeks later.
The dosing of the oral ketamine was 25 mg twice a day with or without sertraline (150 mg/d)/ Antidepressant ratings were lower in the ketamine group starting at week 2 onward. Response rates were greater in the ketamine group, but remission rates did not differ from placebo (22% v 15% respectively)
Conclusions of this study:
Early improvement was significantly greater in the oral ketamine group with Sertraline (85.4%) compared to the placebo group (42.5%). Oral ketamine may be considered as suitable adjuvant to sertraline in relieving depressive symptoms.
A recent systematic review demonstrated that oral ketamine can improve depression symptoms, but remission rates and response rates were marginal compared to placebo. IV ketamine, however, has robust and rapid effects on depression.

In this review, 890 studies were screened, with 10 studies included in the systematic review. Three randomized controlled trials with 160 total patients were included. They found:
- A significant antidepressant effect of oral ketamine.
- Remission rates and response rates were marginal relative to placebo at endpoints.
- Oral ketamine antidepressant effects seemed to take effect in the 2nd week (significant result).
- No significant differences in the overall side-effects between oral ketamine and the placebo group
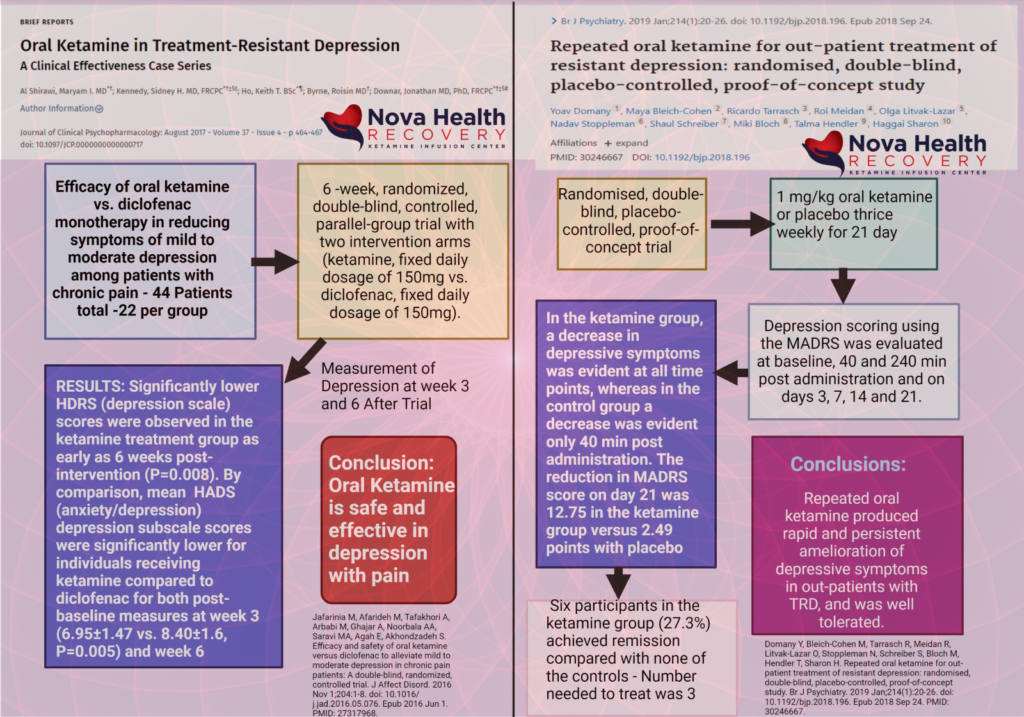
Other Randomized controlled trials have demonstrated efficacy with oral ketamine:(3)

In the RCT with Domany et al. (2109) 41 patients were studied. Oral ketamine (1 mg/kg) or placebo was used. Doses were three times per week.
Results: Ketamine was superior to placebo. At the treatment endpoint, the mean Montgomery-Asberg Depression Rating Scale scores dropped from 33.4 to 20.7 in the ketamine group and from 30.0 to 27.5 in the placebo group.
Response (32% vs 6%) and remission (27% vs 0%) rates were also higher with ketamine than with placebo.

What doses are used in oral ketamine for depression and suicidality?
Doses of oral ketamine have ranged from 0.25 to 7 mg/kg and from 50 mg per occasion to 300 mg per occasion in multiple daily dosing, daily dosing, and intermittent dosing schedules. Periods of up to 3 years have been studied with oral ketamine.
Oral ketamine was well tolerated in all studies. There was no misuse, dose escalation, craving, adverse effects, or dependence reported in these studies.
Oral ketamine is cheaper, easily performed, and has few side-effects. However, it may take a few weeks to see the results of the antidepressant and anti-suicidal effects.
Oral ketamine is poorly absorbed and tastes terrible.
Titration of the oral dose to efficacy needs to be done and varies from person to person due to variable drug absorption.
There has been no misuse of oral ketamine in any study groups for the RCT even though the medication was administered at home.
- Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyama T, Yamamura Y, Yamada Y, Iga T. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003 Jan;24(1):37-43. doi: 10.1002/bdd.336. PMID: 12516077.
- Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982 May;71(5):539-42. doi: 10.1002/jps.2600710516. PMID: 7097501.
- Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, Schreiber S, Bloch M, Hendler T, Sharon H. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. 2019 Jan;214(1):20-26. doi: 10.1192/bjp.2018.196. Epub 2018 Sep 24. PMID: 30246667.
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000 Feb 15;47(4):351-4. doi: 10.1016/s0006-3223(99)00230-9. PMID: 10686270.
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006 Aug;63(8):856-64. doi: 10.1001/archpsyc.63.8.856. PMID: 16894061.
